To mark World Parkinson's Day 2024, let’s put the spotlight on research carried out in Luxembourg to unravel the genetic basis of the disease.
Parkinson's disease affects more than 10 million people worldwide, making it the second most common neurodegenerative disorder after Alzheimer's. Within Western societies, Parkinson’s is the fastest growing neurodegenerative disease and the number of cases is expected to double by 2050, placing an increasing burden on healthcare systems worldwide.
More than 30% of Parkinson's cases are known to have a genetic contribution, however only a few are the result of a single mutation. This means that the majority of cases are classified as idiopathic: the disease is likely caused by a combination of several genetic and environmental factors. Researchers at the National Centre for Excellence in Research on Parkinson's Disease (NCER-PD), together with national and international collaborators, are tirelessly working towards identifying these complex underlying causes.
The National Centre of Excellence in Research on Parkinson's Disease (NCER-PD) is a research programme that brings together the main biomedical research institutes in Luxembourg: the Luxembourg Centre for Systems Biomedicine (LCSB) of the University of Luxembourg, the Luxembourg Institute of Health (LIH) and the Centre Hospitalier de Luxembourg (CHL), in collaboration with the Laboratoire National de Santé (LNS). NCER-PD aims to advance the understanding of Parkinson's disease in order to improve the diagnosis and treatment. The participation of people with different forms of Parkinson's disease and an equal number of healthy controls in Luxembourg and the Greater Region is central to the programme.
The genetic make-up of Parkinson’s disease in Luxembourg
Looking more closely at the genetic underpinnings of the disease is one of the many approaches the researchers are taking to achieve this goal. After years of collecting samples from more than 2000 participants of the Luxembourg Parkinson's Study, including people with Parkinson's and healthy controls, the NCER-PD team has now analysed the complete genetic make-up of most of them.
"Such genetic analyses are essential to define subgroups within people with Parkinson’s disease. It allows us to look closely at already known genetic risk factors but also to detect new variants that might influence the onset or progression of the disease," explains Prof. Rejko Krüger, coordinator of NCER-PD. "With this first large-scale genetic screening conducted in Luxembourg, we identified a major genetic cause explaining more than 12% of Parkinson’s cases in the country, providing new avenues for clinical trials and prevention," he adds.
Detecting genetic variations through DNA screening
To detect genetic mutations linked to the disease, the DNA from blood samples donated by study participants was analysed. The DNA was extracted from the samples and amplified through several rounds of PCR (see box). This amplified DNA was then applied to a chip with thousands of microscopic wells, each containing short pieces of synthetic DNA. The amplified DNA bound to these short pieces and the application of fluorescent labels allowed researchers to discover single variations in the donors genetic code by simply observing the fluorescent light signal of each well.
Using the data from this genetic analysis, NCER-PD researchers found that the percentage of people within the cohort harbouring rare genetic variants that could be linked to Parkinson's disease is similar to what has been reported in other European countries.
DNA is amplified in a process called polymerase chain reaction or PCR for short. It consists of multiple rounds of copying of DNA, resulting in an exponential accumulation of DNA molecules from a tiny sample. Applications of PCR include biomedical research, pathogen detection in medicine and food safety, and criminal forensics.
Distribution of genes harboring mutations within the Luxembourg Parkinson cohort
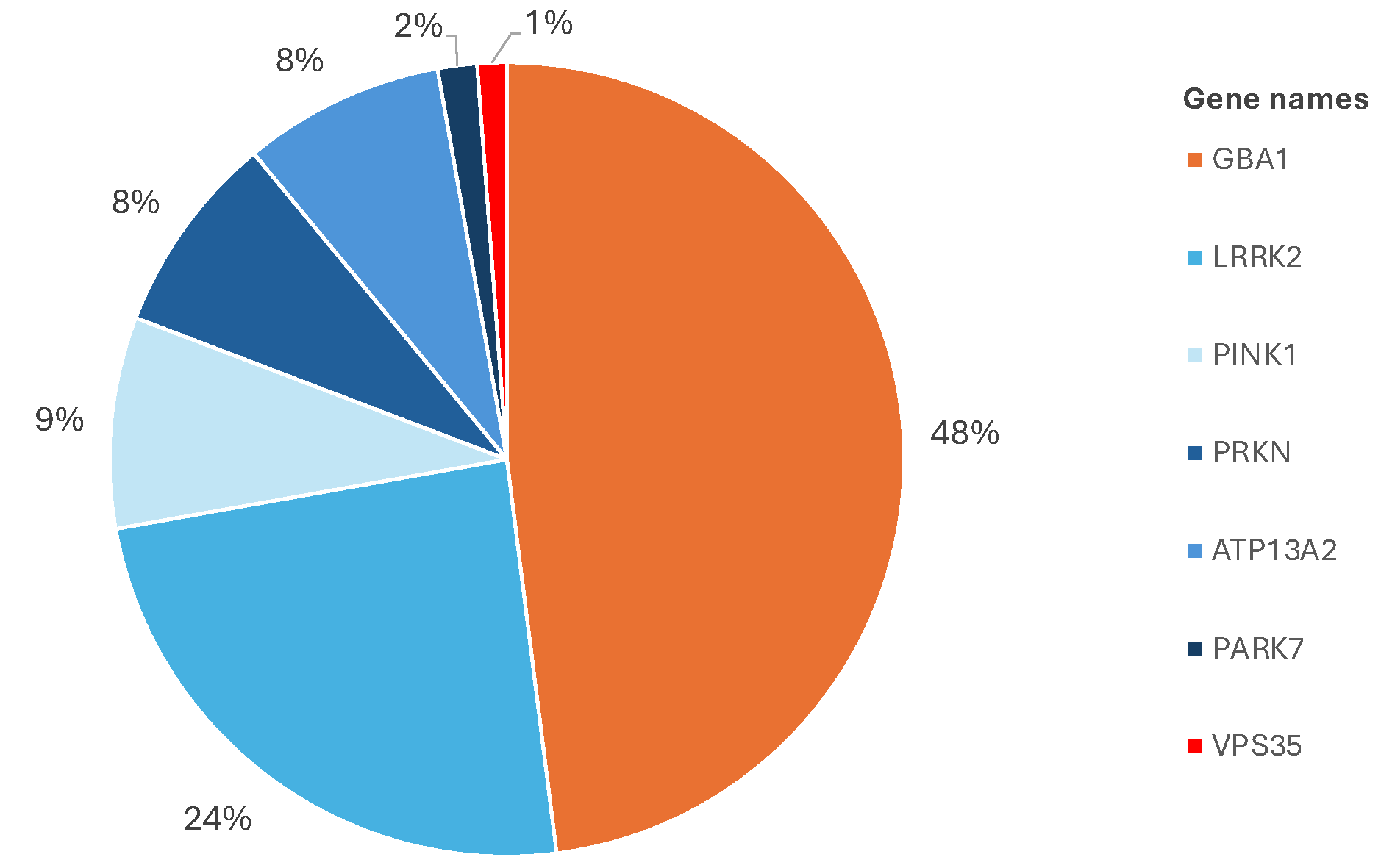
The researchers were also able to confirm that, in addition to the known high-risk single gene variants, several small genetic mutations that are trivial on their own can jointly result in a significantly higher risk for the onset of the disease when they accumulate. How the disease develops from such an accumulation of genetic mutations and how this process can be prevented is the subject of further research.
Focus on the GBA1 gene
The analysis of the entire genetic make-up of the Luxembourg Parkinson's Study cohort lays the groundwork for researchers to look more closely at individual risk variants. Many of these already known genetic variants lie within the GBA1 gene, which contains the building instructions for the enzyme beta-glucocerebrosidase. This protein is responsible for breaking down other faulty proteins. In Parkinson’s disease, the dysfunction of this enzyme promotes the accumulation and aggregation of the misfolded alpha-synuclein protein, which leads to the death of neurons.
Although variations in the GBA1 gene are known to be one of the most important genetic risk factors for developing Parkinson's disease, the exact contribution of all the different mutations that exist is not yet fully understood. It even turns out that the different mutations can be grouped into different categories with different clinical outcomes. To get a more detailed overview of the genetic landscape of the GBA1 gene in the Luxembourgish population, the NCER-PD researchers used a new and very precise method of gene analysis. Unlike the microchip assay used previously, this new technique reads every single nucleotide, the building blocks of DNA, on long stretches of the GBA1 gene. This means that the entire gene was deciphered in detail. This is the first time this technique has been applied to the GBA1 gene worldwide.
"We have not only confirmed the distribution of known variants in our study cohort but we have also discovered new variants that may differently influence disease progression," said Dr Patrick May from the LCSB Bioinformatics Core, who led the analysis of the genetic data. "These new variants are likely to be present in other populations and their potential influence on disease onset and progression will now be further investigated," he adds.
Personalised treatment trials based on genetic analysis
Alongside providing the basis for further genetic research, this genetic analysis has concrete benefits for participants of the Luxembourg Parkinson's Study. Based on their specific genetic background, subgroups of patients can be invited to participate in clinical trials tailored to their specific form of Parkinson’s disease, allowing the development new treatment options that may slow down or even halt disease progression. The first clinical trials in Parkinson's patients with mutations in the GBA1 gene are currently conducted in Italy, aiming at restoring the impaired function of the encoded enzyme and slowing down the premature ageing of cells.
"Thanks to a better understanding of the genetic basis of the disease, we expect that such subgroup-specific treatments will soon move from research to clinical practice. We will launch a first clinical trial specifically for people with Parkinson’s disease due to GBA1 mutations in autumn in Luxembourg," explains Prof. Krüger. "On the long-term, this could mean tailoring treatment for each person with Parkinson's disease, based on their unique genetic makeup but also their environment and lifestyle."
References:
Landoulsi Z, Pachchek S, Bobbili DR, Pavelka L, May P, Krüger R and the NCER-PD Consortium, Genetic landscape of Parkinson’s disease and related diseases in Luxembourg, Frontiers in Aging Neuroscience (2023).
Pachchek, S., Landoulsi, Z., Pavelka, L. et al., Accurate long-read sequencing identified GBA1 as major risk factor in the Luxembourgish Parkinson’s study, npj Parkinson's Disease (2023).




