A first clinical trial in Luxembourg to assess novel drug delivery method is looking for participants
The Luxembourg Institute of Health (LIH) and the Centre Hospitalier de Luxembourg (CHL) announce the launch of “SCOL” (Study of Continuous Oral Levodopa), a unique international clinical trial aiming to assess the safety, tolerability and efficacy of the new DopaFuse System for the continuous oral delivery of levodopa to better treat the symptoms of Parkinson’s Disease (PD). The clinical trial is conducted concomitantly in Italy, Spain and Luxembourg and has the potential to revolutionise the treatment of PD by facilitating drug delivery and reducing side effects.
Preventing fluctuations in levodopa levels
Parkinson’s Disease (PD) is a progressive neurodegenerative disorder that affects predominantly dopaminergic neurons, resulting in declining dopamine levels in the brain and ensuing symptoms such as shaking, stiffness, difficulty with walking, balance and coordination. As still no cure is available, virtually all patients undergo efficient symptomatic treatment with levodopa, which restores dopamine levels thereby controlling some of the typical symptoms. Nevertheless, chronic levodopa treatment through intermittently delivered oral doses is associated with medium-term motor complications, while its currently available continuous intra-intestinal delivery − though entailing a reduction in motor complications − requires invasive surgery with potential related adverse effects.
“Given the side-effects of the current levodopa delivery systems, an alternative method to administer the treatment continuously and in a non-invasive manner while minimising motor side effects remains an important unmet medical need in the treatment of Parkinson’s Disease”, states Dr Guy Berchem, Deputy Director of Research at CHL.
The SCOL clinical trial
SCOL aims to evaluate whether the novel DopaFuse System, developed by the pharmaceutical company SynAgile, can reduce the fluctuation of levodopa levels in the blood, compared to the standard intermittent oral delivery of levodopa tablets. The clinical trial will also assess whether the system is safe, well tolerated and effective in relieving motor symptoms.
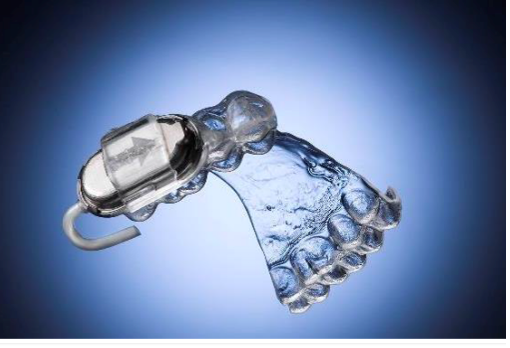
The DopaFuse Delivery System is a non-invasive, intra-oral system that continuously releases a levodopa paste at a controlled rate directly at the back of the patient’s mouth. It consists of a dental retainer, its case and a single-use drug container.
How can you participate?
The SCOL trial will recruit a total of 30 patients across the 3 participating countries. The LIH and the CHL are currently looking to recruit 10 patients with Parkinson’s disease. Individuals above the age of 30 with a confirmed PD diagnosis and a good response to levodopa are eligible to participate. Participation in the study will last 29 days. This clinical trial is completely independent from the National Centre of Excellence in Research on Parkinson’s Disease (NCER-PD) but of course participants of the Luxembourg Parkinson’s Study are invited to join.
At the start of the study, participants will be hospitalised at the CHL for 3 days to replace their previous therapy with standard levodopa tablets with DopaFuse treatment. The participants will subsequently pursue the therapy from home until day 14, before being hospitalised for another day for additional treatment evaluations. Safety assessments and follow-ups will then take place at home until the end of the trial. Regular monitoring visits are foreseen throughout the duration of the study at CHL, with the assistance of the LIH Transversal Translational Medicine (TTM) and Clinical and Epidemiological Investigation Center (CIEC) teams. Clinical data and blood samples will also be collected as part of the trial.
What is the difference between a clinical trial like SCOL and a clinical research study like NCER-PD?
Clinical research is defined as all medical research performed on humans. It aims to improve the knowledge of diseases in order to improve medical care for the patients in the long run. The Luxembourg Parkinson’s Study of NCER-PD is one of the largest clinical research studies in Luxembourg. By investigating 800 Parkinson’s patients and 800 control subjects, researchers aim to find new ways for earlier diagnosis and better treatments. Importantly, the Luxembourg Parkinson’s Study is an observational clinical study. This means that no drugs are administered in the course of the study and that patients keep taking the treatment given by their treating physician.
In contrast, clinical trials, often carried out by pharmaceutical companies together with healthcare or academic partners, are the last step in the long research and development process of a new drug. They assess new potential treatments for safety and efficacy. Participants will hence undergo changes in treatment during a clinical trial and be strictly monitored over a defined period of time. The SCOL clinical trial is the first Parkinson’s disease clinical trial in Luxembourg. Its aim is to evaluate if the DopaFuse System can reduce the fluctuation of levodopa levels in the blood compared to standard treatment with Levodopa pills and assess how well participants tolerate the new treatment.
The link to the NCER-PD study programme
As mentioned before, the Luxembourg Parkinson’s Study is an observational clinical study. This study is a joint effort with several national and international partners with the aim of contributing to Parkinson’s disease research in Luxembourg. The Luxembourg Institute of Health (LIH) and the Centre Hospitalier de Luxembourg (CHL), who are partners in the Luxemburg Parkinson’s Study since its beginning, are in charge of the SCOL clinical trial in Luxembourg and will bring their valuable expertise on Parkinson’s disease to this clinical trial.
“The SCOL study is another example of the pioneering clinical research being done in Luxembourg. Indeed, we are one of the first three countries in the world to be trialling this levodopa delivery method, owing our long-standing expertise in PD exemplified by joint initiatives of Luxembourg's research institutions, such as the National Centre for Excellence in Parkinson's Disease Research (NCER-PD). We are confident that our innovaticve approach will allow us to generate meaningful results and contribute to improving treatment outcomes for our PD patients”, concludes Prof Rejko Krüger, principal investigator at the CHL clinical site in Luxembourg, director of Transversal Translational Medicine (TTM) at LIH and coordinator of the NCER-PD programme.
If you are interested in participating, contact the following number: +352 44 11 8359




